The opioid crisis reaches far beyond the estimated 2 million people who are addicted and 130 who die from overdose each day in the U.S. According to the National Institute on Drug Abuse, a baby is born addicted to opioids every 15 minutes, and that is based on data from 2014. Neonatal Abstinence Syndrome (NAS), withdrawal from opioids after … [Read more...]
Heartburn Drug Contaminated with NDMA
The Food and Drug Administration (FDA) has announced that low levels of a potentially dangerous contaminant have been found in Zantac heartburn medication, both the prescription and over-the-counter version of the popular drug taken to treat stomach acid and ulcers. FDA officials report that while the amount of NDMA found in the drug “barely … [Read more...]
New Research May Show Link Between Talcum Powder and Cancer
For years, Johnson & Johnson has been plagued with allegations that its famous baby powder causes ovarian cancer when it’s used in the genital area over long periods of time by women. While Johnson & Johnson has insisted its product is safe, juries across the United States have disagreed and have awarded large sums of money to female … [Read more...]
Dangerous Drug Lawsuit: Truvada
Truvada, marketed by the manufacturer Gilead, is the most popular and oft-prescribed TDF drug designed to treat and prevent human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS). A combination of the chemicals emtricitabine and tenofovir disoproxil fumarate (TDF), Truvada prevents HIV cells from multiplying once those … [Read more...]
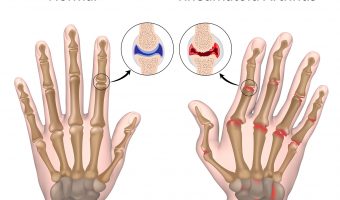
Xeljanz May Cause Blood Clots and Death According to Safety Study
On February 29, 2019, the U.S. Food and Drug Administration (FDA) issued a safety communication warning the public about an increased risk of blood clots and death in patients with rheumatoid arthritis taking a high dose of Xeljanz (tofacitinib). The warning was prompted by a mandatory post-marketing safety trial of the drug. The trial is still … [Read more...]
- « Previous Page
- 1
- 2
- 3
- 4
- 5
- …
- 45
- Next Page »




Recent Comments