Mesothelioma is a type of cancer caused, almost exclusively, by asbestos exposure. As such, it has long been associated with workplace exposure, primarily in industries such as construction, mining and steel work. And it is thought of as a disease that affects older men. Although more men are diagnosed with mesothelioma than women, the gender gap … [Read more...]
Bausch Removed Talc Powder from the Shelves Long Before Johnson & Johnson’s Recall
Johnson & Johnson (J&J) is currently facing thousands of lawsuits alleging the talc in its baby powder causes cancer. After fighting against those claims for years, the company suddenly recalled over 30,000 bottles of baby powder just last month after Food & Drug Administration tests revealed the product contained asbestos. Eight … [Read more...]
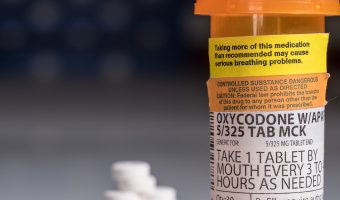
DEA Allowed Increased Oxy Production During Overdose Crisis
On the home page of the Drug Enforcement Administration’s website, the government agency known simply as the “DEA” has published its mission statement as follows: “To enforce the controlled substances laws and regulations of the United States and bring to the criminal and civil justice system of the United States, or any other competent … [Read more...]
New Study Could Prove Link Between Mesothelioma and Talcum Powder
For the past few years, we have heard about a number of lawsuits being filed alleging that baby powder containing talc causes cancer. Last year, Johnson & Johnson was ordered to pay over $4 billion to a group of women who developed ovarian cancer. In 2016, the manufacturing giant was ordered to pay $55 million to a single plaintiff who … [Read more...]
Johnson & Johnson Baby Powder Recalled Due to Asbestos Contamination
On Friday, October 18, 2019, Johnson & Johnson (J&J) announced a recall of 33,000 bottles of its baby powder, due to contamination with asbestos. The recall applies to one lot, lot #22318RB, which was distributed in 2018. If you have a bottle of Johnson’s Baby Powder from this lot, you are advised to discontinue use and contact the company … [Read more...]
- « Previous Page
- 1
- 2
- 3
- 4
- …
- 45
- Next Page »




Recent Comments