On Wednesday, December 14, 2016, the US Food and Drug Administration (FDA) released a safety announcement regarding the risks of repeat usage of anesthesia in children under three. According to several studies, repeated anesthesia use has be associated with developmental disorders such as attention deficit and hyperactivity … [Read more...]
LG Electronics Recalls Portable Air Conditioners Due to Fire Hazard
Arcadia Trading Inc. Recalls Lizard Fish Due to Possible Health Risk
Arcadia Trading Inc. of Brooklyn, NY, is recalling 34 cartons of Lizard Fish because they have the potential to be contaminated with Clostridium botulinum, a bacterium which can cause life-threatening illness or death. Consumers are warned not to consume the product even if it does not look or smell spoiled. … [Read more...]
Ron’s Home Style Foods, Inc. Recalls Tropical Fruit Supreme, Pineapple Nut Delight, and Pistachio Crème Because Of Possible Health Risk
Ron’s Home Style Foods, Inc. of Houston, Texas is recalling Tropical Fruit Supreme, Pineapple Nut Delight, and Pistachio Crème, because it has the potential to be contaminated with Salmonella, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. … [Read more...]
Old Dutch Recalls Various Flavored Potato Chip Products due to Possible Health Risk
Old Dutch Foods Inc. of St. Paul, MN is voluntarily recalling a limited number of Flavored Potato Chip and Tortilla Chip products that were made with milk ingredients supplied by one of its’ secondary seasoning component suppliers Valley Milk Products LLC, due to the potential risk of Salmonella contamination. … [Read more...]
New Hope Mills Expands Recall of Limited Amounts of Crepe Mix as a Precaution Due to Possible Health Risk
Auburn, NY - New Hope Mills is expanding its 12/12/16 voluntary recall for one additional code of its NEW HOPE MILLS Crepe MIX as it has the potential to be contaminated with Salmonella. The ingredient supplier has issued a recall of the bulk milk powder. … [Read more...]
Remington Brand Chainsaws Recalled by MTD Southwest Due to Fire Hazard
Craftsman Brand Chainsaws Recalled by MTD Southwest Due to Fire Hazard
Figi’s Companies Recalls “Christmas Wishes” Tins Due to Choking and Button Battery Ingestion Hazard (Recall Alert)
Ridley Block Operations Announces Voluntary Recall of Ultralyx 24% + 3% Mag Composite
Ridley Block Operations has initiated a voluntary recall of a single batch of its beef cattle feed product, Ultralyx 24% + 3% Mag Composite Block, because it contains elevated levels of non-protein nitrogen (NPN) that may be harmful to beef cattle. … [Read more...]
Thomas Hammer Coffee Roasters Inc. Issues Allergy Alert on Undeclared Milk, Egg, Soy, Wheat in The Cranberry Orange Scone
December 14th, 2016, Thomas Hammer Coffee Roasters Inc. of Spokane, Washington is recalling 52 Cranberry Orange Scones, because they may contain undeclared MILK, EGG, SOY, and WHEAT. People who have an allergy or severe sensitivity to MILK, EGG, SOY, WHEAT run the risk of serious or life-threatening allergic reaction if they consume this product. … [Read more...]
H-E-B Issues Recall On Raw Shelled Pistachios
H-E-B announced today that it has issued a recall for both bulk and packaged raw shelled pistachios. The product is being removed because there is potential it could be contaminated with Salmonella, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune … [Read more...]
Trek Recalls Bicycle Lights Due to Injury Hazard
Linden Cookies Issues Allergy Alert on Undeclared Milk in Mini Chocolate Chip Cookies and 3 Pack Chocolate Chip Cookies
Linden Cookies, Inc. in Congers New York is recalling fully baked Linden’s Chocolate Chip Cookies because they contain undeclared milk. People who have an allergy to milk run the risk of serious or life-threatening allergic reaction if they consume this product. … [Read more...]
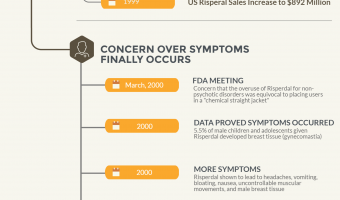
Risperdal Infographic
Risperdal is a drug manufactured by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson. It was developed and originally approved in 1993 to be used as an antipsychotic medication. However, soon after receiving its original FDA-approval, Janssen began to market Risperdal to those who were not originally intended to take the drug; … [Read more...]
- « Previous Page
- 1
- …
- 700
- 701
- 702
- 703
- 704
- …
- 1271
- Next Page »

Recent Comments