Every day, regular people deal with tragedy, illness, and sadness, and most people going through a difficult time endure hardships privately with the support of a few loved ones. Some people, sadly, go through extremely tough times completely alone, suffering in silence and isolation. When someone famous becomes ill, however, it seems the world takes notice and rallies around to offer words of encouragement, healing, and support.
One good thing that has always come from the ill health of celebrities is the increased publicity surrounding the specific condition or disease we had really not previously known about or understood. A celebrity’s illness may also remind us that sickness knows no socioeconomic status.
MRI Poisoning?
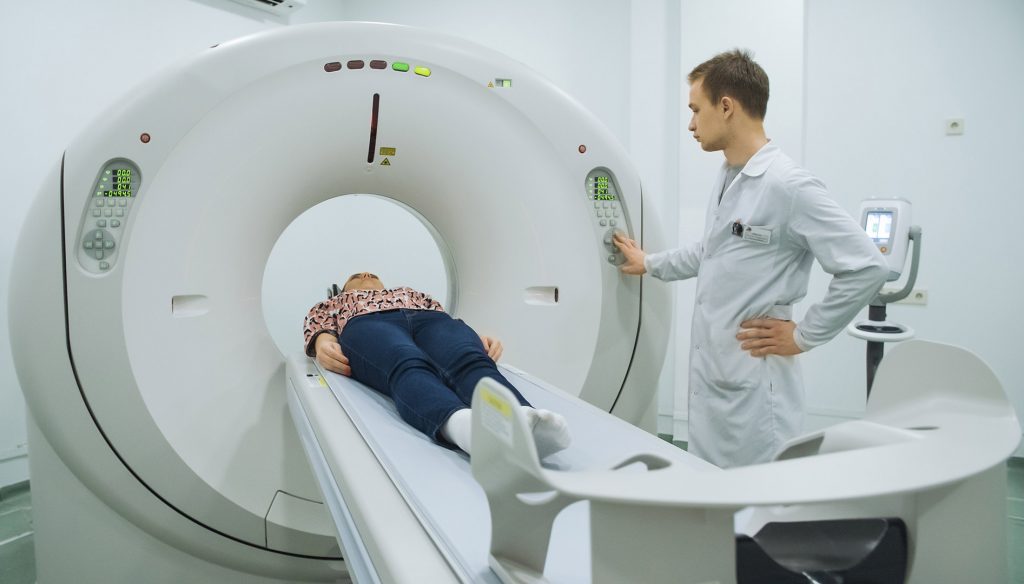
While we are all familiar with cancer, heart disease, dementia, and multiple sclerosis, there are lesser known conditions out there. One example of this is gadolinium toxicity. Chuck Norris’ wife, Gena O’Kelley Norris, is battling one such condition. In 2017, Mrs. Norris filed a gadolinium toxicity lawsuit. She claims that she had a severe, adverse reaction to gadolinium, which was used during an MRI (magnetic resonance imaging) she had years ago.
Gadolinium is a heavy metal commonly injected into a person’s veins before an MRI to increase visual contrast, making internal organs more visible on this type of scan. Gadolinium is used because it was long believed to exit a person’s system just hours after the MRI is completed.
Norris’ lawsuit argues that the metal does not leave the human system shortly after the MRI, and, in fact, can remain in a person’s system for years after the scan. Norris and others have claimed that gadolinium is poisonous and can cause a range of serious side effects. Norris said her symptoms of gadolinium toxicity developed slowly over a period of several years.
She experienced thickening of tissue, burning sensations on her skin, “mind fog,” pain, skin lesions and other symptoms associated with nephrogenic system fibrosis (NSF). NSF is the only condition that linked to gadolinium deposition, has always been mainly associated with patients with kidney failure and kidney disease. Norris’ gadolinium lawsuit claims she developed NSF symptoms, even though she didn’t have kidney problems.
The lawsuit states this is an example of how gadolinium can have negative effects even in patients with normal kidney function, so it can be dangerous to all patients. The lawsuit also says the medical community has not paid enough attention to the very real likelihood that healthy patients can develop gadolinium toxicity and become very sick. A toxicologist near Dallas saw Norris as a patient and says he has seen several other patients with cases similar to hers. He has supported her argument that doctors have not fully investigated the possible dangers of gadolinium in MRI contrast dye.
If you have been exposed to gadolinium via an MRI, and you’ve noticed symptoms similar to the ones described here, please contact a pharmaceutical injury attorney who handles these complex cases.
The NSF Registry at Yake established a point system to distinguish and exaggerate the differences between NSF, Scleromyxedema and Scleroderma. They ignored multiple pleas for Marci Jacobs who died of Gadolinium induced Systemic Sclerosis, Scleroderma. I’m hoping to charge the for the murder if Dr. John Walk who died quickly for six injections and Gadolinium induced Scleroderma this June 2018. This is not Med mal. This is. Breach of Helsinki and manslaughter.
I am allergic to Gadolinium. It gave me a migraine for a month. My doctor and I tried teverthing to get the pain to stop. After a month she suggested Botox and it worked. $100 well spent.