Leclerc Foods has initiated a limited voluntary recall of a single lot of Fit & Active Chocolatey Chip Protein Meal Bars packages with UPC Code 41498-18695 and only affected products with a “best by” date of May 24, 2018. The recall was initiated as a precautionary measure after a small piece of yellow plastic was discovered by a … [Read more...]
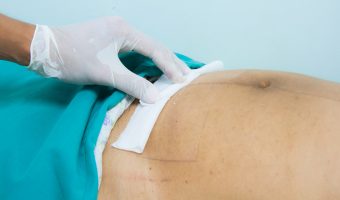
Covidien Parietex Hernia Mesh Complications and Lawsuit
Covidien surgical mesh products have been on the market for several years. They were approved by the Food and Drug Administration (FDA) for use in hernia repair procedures. However, they have been linked to various complications in patients. Complications linked to Covidien Hernia Mesh The FDA issued warnings to health practitioners and the … [Read more...]
Sun Pharmaceutical Industries Inc. Issues Voluntary Nationwide Recall of Riomet® (Metformin Hydrochloride Oral Solution) Manufactured by a Contract Manufacturer due to Microbial Contamination
Sun Pharmaceutical Industries, Inc. (SPII), a wholly owned subsidiary of Sun Pharmaceutical Industries Ltd. (Reuters: SUN.BO, Bloomberg: SUNP IN, NSE: SUNPHARMA, BSE: 524715, “Sun Pharma” including its subsidiaries and/or associate companies) is voluntarily recalling two lots of Riomet® (Metformin Hydrochloride Oral Solution), 500 … [Read more...]
EAST ( 17V684000 )
Dated: OCT 27, 2017 East Manufacturing Corp. (East) is recalling certain 2013-2018 Frameless Dump Hybrid trailers. These trailers have draft arm tubing that may crack and fail. … [Read more...]
HONDA ( 17V725000 )
Dated: NOV 17, 2017 Honda (American Honda Motor Co.) is recalling certain 2011-2017 Honda Odyssey vehicles. The second row outboard seats can slide sideways to one of two positions. If a seat is placed between either o... … [Read more...]
MCI ( 17V687000 )
Dated: OCT 30, 2017 Motor Coach Industries (MCI) is recalling certain 2018 MCI J4500 buses. The primary air line was incorrectly hooked to the secondary air pressure gauge and the secondary air line was hooked to the pr... … [Read more...]
ELDORADO ( 17V685000 )
Dated: OCT 27, 2017 Eldorado National-California, Inc. (Eldorado National) is recalling certain 2017 Axess, E-Z Rider, XHF, and Arrivo buses, equipped with certain Recaro Commercial Bus Driver Seats, models Ergo R, Ergo ... … [Read more...]
KZRV ( 17V688000 )
Dated: OCT 31, 2017 KZRV, L.P. (KZRV) is recalling certain 2017-2018 Connect recreational trailers equipped with an outside kitchen. The outlet for outside kitchen area does not have Ground Fault Circuit Interrupter (GF... … [Read more...]
Food Co. Issues Allergy Alert on Undeclared Allergen (E.G. Milk) in Product
Colorado Nut Company of Denver, CO is recalling Cashew Cranberry Cherry Jubilee, Oat Bran Nutty Crunch, Honey Nutty Granola, Peanut Delight, and Frontier Trail Mix, because they may contain undeclared Milk. People who have an allergy or severe sensitivity to Milk run the risk of serious or life-threatening allergic reaction if they consume these … [Read more...]
TREMCAR ( 17V680000 )
Dated: OCT 26, 2017 Tremcar is recalling certain 2008-2017 Hutchinson and Tremcar 406 B-train Pups, 2012-2017 Hutchinson and Tremcar 406 2 Axle Semi-Trailers, 2015-2017 Tremcar 406 3 Axle Semi-Trailers, and 2013 Tremcar ... … [Read more...]
HONDA ( 17V681000 )
Dated: OCT 26, 2017 Honda (American Honda Motor Co.) is recalling certain 2017 Honda CBR1000RR and CBR1000RR SP motorcycles. There may be a gap between the fuel tank cap seal and the fuel filler neck which can allow wat... … [Read more...]
JAGUAR ( 17V678000 )
Dated: OCT 26, 2017 Jaguar Land Rover North America, LLC (Jaguar) is recalling certain 2017-2018 Jaguar XF and F-Pace, 2016-2017 Jaguar XJ and 2018 Jaguar XE vehicles. The instrument cluster (IC) may intermittently go b... … [Read more...]
LAND ROVER ( 17V679000 )
Dated: OCT 26, 2017 Jaguar Land Rover North America, LLC (Land Rover) is recalling certain 2017 Land Rover Range Rover and Range Rover Sport vehicles. The instrument cluster (IC) may intermittently go blank. … [Read more...]
FORD ( 17V671000 )
Dated: OCT 24, 2017 Ford Motor Company (Ford) is recalling certain 2017 F-150 vehicles equipped with ten-speed transmissions. The pin that attaches the transmission shift linkage to the transmission may come out, preven... … [Read more...]
Goal Zero Recalls Solar Powered Charging Stations Due to Impact Hazard (Recall Alert)
The weld connecting the top and bottom posts can fail and allow the top post to fall, posing an impact hazard to nearby consumers. … [Read more...]
- « Previous Page
- 1
- …
- 618
- 619
- 620
- 621
- 622
- …
- 1271
- Next Page »



Recent Comments