The speaker’s battery can become overcharged and burst, posing an impact hazard. … [Read more...]
B&B Acquisition Recalls Wintergreen Essential Oils Due to Failure to Meet Child Resistant Packaging Requirements; Risk of Poisoning
The product contains the substance methyl salicylate, which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the product is not child resistant, posing a poisoning risk if the contents are swallowed by young children. … [Read more...]
Deck Source Recalls Cutek Proclean Due to Violation of FHSA Labeling Requirements; Risk of Poisoning
The product contains the hazardous substance oxalic acid. The label on the product violates the Federal Hazardous Substance Act (FHSA) by omitting the word “poison” and printing the mandatory hazard statement for poisonous chemicals on the back of the label instead of the front, posing a risk of poisoning if swallowed by children. … [Read more...]
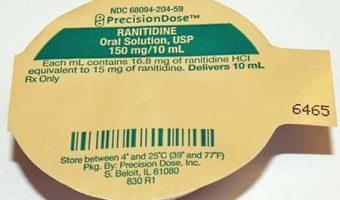
Precision Dose Inc. Issues Voluntary Nationwide Recall of Ranitidine Oral Solution, USP 150 mg/10 mL Due to Possible Presence of N-nitrosodimethylamine (NDMA) Impurity.
Summary Company Announcement Date: November 19, 2019 FDA Publish Date: November 19, 2019 Product Type: Drugs Reason for Announcement: Recall Reason Description Potential presence of N-Nitrosodimethylamine (NDMA) above levels established by the FDA Company Name: Precision Dose Inc. Brand Name: Brand Name(s) PrecisionDose Product … [Read more...]
MAZDA ( 19V781000 )
Dated: OCT 31, 2019 Mazda North American Operations (Mazda) is recalling certain 2003-2008 Mazda6, 2004-2005 MPV, 2004 RX-8, and 2006-2007 Mazdaspeed6 vehicles. These vehicles had their passenger frontal air bag inflato... … [Read more...]
MAZDA ( 19V782000 )
Dated: OCT 31, 2019 Mazda North American Operations (Mazda) is recalling certain 2007-2012 CX-7, CX-9, and 2009-2012 Maza6 vehicles. These vehicles had their passenger frontal air bag inflators previously replaced under... … [Read more...]
FORD ( 19V766000 )
Dated: OCT 25, 2019 Ford Motor Company (Ford) is recalling certain 2019 Edge vehicles. The driver-side seat belt pretensioner anchor may have been improperly crimped, possibly resulting in the seat belt webbing detachin... … [Read more...]
LINCOLN ( 19V773000 )
Dated: OCT 28, 2019 Ford Motor Company (Ford) is recalling certain 2019 Expedition and Lincoln Navigator vehicles. The rear suspension toe link fasteners may not have been properly tightened to the frame, possibly allow... … [Read more...]
United National Closeout Stores Recalls Isometric Exercise Devices Due to Projectile Hazard; Devices Sold at Burlington Stores After 2014 Recall
The handle grips on each end can break during use and cause parts to be forcefully ejected from the shaft, posing a risk of injury from impact to the user or bystander. … [Read more...]
Flying Tiger Copenhagen Recalls Crocodile Candleholders Due to Fire Hazard
The candleholders are flammable and can catch on fire, posing a fire hazard when the candle burns down. … [Read more...]
Select Varieties of Breakstone’s Cottage Cheese are Voluntarily Being Recalled Due to Potential Presence of Foreign Material
Summary Company Announcement Date: November 15, 2019 FDA Publish Date: November 15, 2019 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Potential presence of plastic and metal Company Name: Kraft Heinz Foods Company Brand Name: Brand Name(s) Breakstone’s Product Description: Product … [Read more...]
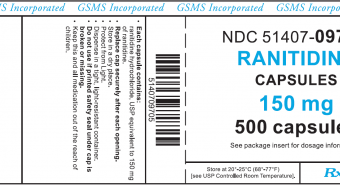
Golden State Medical Supply, Inc. Issues a Voluntary Nationwide Recall of Ranitidine Hydrochloride 150mg and 300mg Capsules (Manufactured by Novitium Pharma LLC) Due to an Elevated Amount of Unexpected Impurity, N-Nitrosodimethylamine (NDMA)
Summary Company Announcement Date: November 15, 2019 FDA Publish Date: November 15, 2019 Product Type: Drugs Reason for Announcement: Recall Reason Description Potential presence of N-Nitrosodimethylamine (NDMA) above levels established by the FDA Company Name: GSMS, Inc. Brand Name: Brand Name(s) GSMS Incorporated Product … [Read more...]
Bausch Removed Talc Powder from the Shelves Long Before Johnson & Johnson’s Recall
Johnson & Johnson (J&J) is currently facing thousands of lawsuits alleging the talc in its baby powder causes cancer. After fighting against those claims for years, the company suddenly recalled over 30,000 bottles of baby powder just last month after Food & Drug Administration tests revealed the product contained asbestos. Eight … [Read more...]
MAZDA ( 19V770000 )
Dated: OCT 28, 2019 Mazda North American Operations (Mazda) is recalling certain 2019 CX-9 vehicles equipped with Sumitomo Falken ZIEX CT50 A/S tires, size P255/50R20 104V, having date code 1619. An incorrect rubber com... … [Read more...]
PREVOST ( 19V777000 )
Dated: OCT 29, 2019 Prevost Cars, Inc. (Prevost) is recalling certain 2020 X3-45 VIP, X3-45 VIP Entertainer, X3-45 Passenger, H3-45 VIP, and H3-45 Passenger coaches. Due to a missing component, the driver may not be abl... … [Read more...]
- « Previous Page
- 1
- …
- 406
- 407
- 408
- 409
- 410
- …
- 1271
- Next Page »




Recent Comments