Summary Company Announcement Date: January 03, 2025 FDA Publish Date: January 03, 2025 Product Type: Food & Beverages … [Read more...]
Euqee Wintergreen Essential Oils Recalled Due to Risk of Poisoning; Violation of the Poison Prevention Packaging Act; Sold Exclusively on Amazon.com by DLWL-US
The essential oil contains methyl salicylate, which must be in child-resistant packaging, as required by the Poison Prevention Packaging Act (PPPA). The packaging for the products is not child-resistant, posing a risk of poisoning if the contents are swallowed by young children. … [Read more...]
Kawasaki Motors USA Recalls John Deere ZTrak Zero Turn Mowers with Kawasaki Engines Due to Fire and Burn Hazards
The voltage regulator in the mower’s engine can fail during use or while the product is being stored, causing the engine to overheat, posing fire and burn hazards. … [Read more...]
Yaomiao Children’s Jewelry Sets Recalled Due to Risk of Lead and Cadmium Poisoning; Violations of the Federal Lead Content Ban and Federal Hazardous Substances Act; Sold Exclusively on Amazon by LordRoads
The recalled jewelry sets contain levels of lead that exceed the federal lead content ban and levels of cadmium that are prohibited in children’s products by the Federal Hazardous Substances Act. Lead and cadmium are toxic if ingested by young children and can cause adverse health effects. … [Read more...]
Braga Fresh Issues Voluntary and Precautionary Advisory Due to Possible Health Risk
Summary Company Announcement Date: December 27, 2024 FDA Publish Date: December 31, 2024 Product Type: Food & Beverages … [Read more...]
Gardners Candies Issues Allergy Alert on Undeclared Tree Nuts in Cappuccino Meltaway® Bars and Gardners Meltaway Treat Boxes Containing Cappuccino Meltaway Bars
Summary Company Announcement Date: December 27, 2024 FDA Publish Date: December 27, 2024 Product Type: Food & Beverages … [Read more...]
Black Diamond Equipment Recalls PIEPS Pro IPS Avalanche Transceivers Due to Risk of Loss of Emergency Communications
The battery compartment within the avalanche transceiver could not have enough contact force to supply power. In some cases, the transceiver will fail to power on or cease operating during use. It would make it impossible to locate a victim in an avalanche emergency situation, which could result in serious injury or death. … [Read more...]
GIKPAL Dressers Recalled Due to Tip-Over and Entrapment Hazards; Violations of Federal Regulation for Clothing Storage Units; Sold Exclusively on Walmart.com by ONME Direct
The recalled dressers are unstable if they are not anchored to the wall, posing serious tip-over and entrapment hazards that can result in injuries or death to children. The dressers violate performance and warning label requirements of the STURDY Act. … [Read more...]
Precor Recalls Resolute™ Cable Multi-Station Exercise Equipment Due to Impact Injury Hazard
The pop-pin can fail to fully engage in the exercise equipment’s vertical rail and cause the pulley carriage to drop unexpectedly during use, posing an impact injury hazard to users. … [Read more...]
BCI Burke Recalls Playground Merry-Go-Rounds Due to Crush Hazard
The space between the rotating platform and the stationary outer rim can leave a gap that narrows too much, posing a crushing hazard to children. … [Read more...]
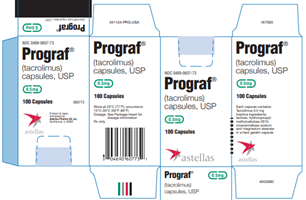
Astellas Pharma US, Inc. Issues Voluntary Nationwide Recall of One Lot of PROGRAF® 0.5mg (Tacrolimus) and One Lot of ASTAGRAF XL® 0.5mg (Tacrolimus Extended-Release Capsules) Because Bottles Shipped to U.S. May Contain Empty Capsules
Summary Company Announcement Date: December 23, 2024 FDA Publish Date: December 24, 2024 Product Type: Drugs … [Read more...]
Alcon Laboratories Issues Voluntary Nationwide Recall of One (1) Lot of Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, 25 count (Lot 10101), Due to Fungal Contamination
Summary Company Announcement Date: December 21, 2024 FDA Publish Date: December 23, 2024 Product Type: Drugs … [Read more...]
Lidl Recalls Taste of Deutschland Buttered Vegetables Due to Undeclared Milk Allergens
Summary Company Announcement Date: December 20, 2024 FDA Publish Date: December 20, 2024 Product Type: Food & Beverages … [Read more...]
Endo USA, Inc. Issues Voluntary, Nationwide Recall of Adrenalin® Chloride Solution (EPINEPHrine Nasal Solution, USP) Due to the Potential for Administration Errors
Summary Company Announcement Date: December 20, 2024 FDA Publish Date: December 20, 2024 Product Type: Drugs … [Read more...]
Recall of Jose Madrid Salsa Chipotle Con Queso
Summary Company Announcement Date: December 20, 2024 FDA Publish Date: December 20, 2024 Product Type: Food & Beverages … [Read more...]
- « Previous Page
- 1
- …
- 26
- 27
- 28
- 29
- 30
- …
- 1271
- Next Page »






Recent Comments