The vehicles were manufactured without copper seal washers on the turbocharger’s oil supply line, which can result in an oil leak, posing a fire hazard. … [Read more...]
PORSCHE ( 21V318000 )
Dated: MAY 05, 2021 Porsche Cars North America, Inc. (Porsche) is recalling certain 2021 Cayenne GTS, Cayenne GTS Coupe, 2020-2021 Cayenne Coupe, Cayenne S Coupe, Cayenne E-Hybrid Coupe vehicles. The passenger seat back... … [Read more...]
JAYCO ( 21V317000 )
Dated: MAY 05, 2021 Jayco, Inc. (Jayco) is recalling certain 2021 Travel Trailer Jay Feather Micro trailers. The spare tire carrier could break at the bend radius or gusset. … [Read more...]
BLUE BIRD ( 21V312000 )
Dated: MAY 04, 2021 Blue Bird Body Company (Blue Bird) is recalling certain 2018-2022 All American buses. The sealing washer may not seat correctly in the pilot bore holes, allowing the high pressure fuel rail assembly ... … [Read more...]
BLUE BIRD ( 21V313000 )
Dated: MAY 04, 2021 Blue Bird Body Company (Blue Bird) is recalling certain 2018-2022 All American vehicles. The sealing washer may not seat correctly in the pilot bore holes, allowing the high pressure fuel rail assemb... … [Read more...]
DUCATI ( 21V315000 )
Dated: MAY 04, 2021 Ducati North America (Ducati) is recalling certain 2017-2020 Monster 797, Monster 1200, Supersport, 2016-2020 XDiavel, and 2018-2020 Monster 821 motorcycles. Air may permeate the rear bake system. … [Read more...]
JOLLY TIME® Pop Corn Issues Allergy Alert on Undeclared Milk in Healthy Pop® Kettle Corn 100’s (4 count)
Summary Company Announcement Date: May 12, 2021 FDA Publish Date: May 13, 2021 Product Type: Food & Beverages … [Read more...]
Global Sanitizers Issues Voluntary Nationwide Recall of Medically Minded Hand Sanitizer Due to Presence of Undeclared Methanol
Summary Company Announcement Date: May 12, 2021 FDA Publish Date: May 13, 2021 Product Type: Drugs … [Read more...]
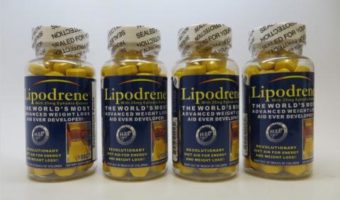
Hi Tech Pharmaceuticals Issues Voluntary Recall on Lipodrene w/25mg Ephedra Extract Dietary Supplement Due to the Presence of 1,4-dimethylamylamine (DMAA)
Summary Company Announcement Date: May 12, 2021 FDA Publish Date: May 12, 2021 Product Type: Food & Beverages … [Read more...]
Briggs & Stratton Recalls YTL, Longli and TIYA Log Splitters with Briggs & Stratton® Engines Due to Injury Hazard
An incorrect engine ignition coil and flywheel can lead to hard starting and engine kick back, posing an injury hazard to the operator. … [Read more...]
K & M International Recalls Slap Watches Due to Coin Cell Battery Ingestion and Choking Hazards
The coin cell battery inside the slap watches can fall out, posing battery ingestion and choking hazards to young children. … [Read more...]
Dibar Nutricional S. de R.L. De C.V. Issues Voluntary Nationwide Recall of DIBAR Labs Hand Sanitizer Due to the Presence of Methanol (Wood Alcohol)
Summary Company Announcement Date: May 11, 2021 FDA Publish Date: May 12, 2021 Product Type: Drugs … [Read more...]
Dash Xclusive Issues Voluntary Nationwide Recall of Imperia Elita Vitaccino Coffee Due to the Presence of Undeclared Sibutramine and Fluoxetine
Summary Company Announcement Date: May 11, 2021 FDA Publish Date: May 11, 2021 Product Type: Dietary Supplements … [Read more...]
ARCIMOTO ( 21V314000 )
Dated: MAY 01, 2020 Arcimoto Inc (Arcimoto) is recalling certain 2019-2021 FUV, 2020 Deliverator, 2021 Rapid Responder motorcycles. The seat belt retractor spool sleeve may be cracked, allowing broken pieces to block th... … [Read more...]
ARCIMOTO ( 21V311000 )
Dated: MAY 04, 2021 Arcimoto Inc (Arcimoto) is recalling certain 2019-2021 FUV, 2021 Rapid Responder, 2020 Deliverator, 2020-2021 Roadster. Due to a software error, communication between inverters may time out, possibl... … [Read more...]
- « Previous Page
- 1
- …
- 262
- 263
- 264
- 265
- 266
- …
- 1271
- Next Page »





Recent Comments