Summary
- Company Announcement Date:
- September 11, 2023
- FDA Publish Date:
- September 11, 2023
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Crystal formation which could potentially result in incorrect dosing
- Company Name:
- Novartis Pharmaceuticals Corporation
- Brand Name:
-
Brand Name(s)
- Product Description:
-
Product Description
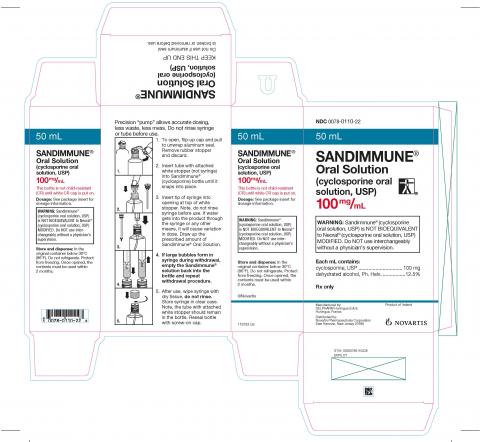
Sandimunne Oral Solution (cyclosporine oral solution, USP) 100 mg/mL
Company Announcement
FOR IMMEDIATE RELEASE – September 11, 2023 – East Hanover, NJ — Novartis is conducting a voluntary nationwide recall at the consumer level of one lot of its Sandimmune® Oral Solution (cyclosporine oral solution, USP), 100 mg/mL in the US due to crystal formation observed in some bottles, which could potentially result in incorrect dosing. No other Sandimmune formulations are impacted.
Sandimmune® Oral Solution (cyclosporine oral solution, USP), 100 mg/mL, packaged in 50 mL bottles, is indicated for the prophylaxis of organ rejection in kidney, liver, and heart allogeneic transplants. The drug may also be used in the treatment of chronic rejection in patients previously treated with other immunosuppressive agents.
Risk Statement: Crystallization of cyclosporine in Sandimmune Oral Solution is likely to result in non-uniform distribution of the cyclosporine in the product, resulting in under-dosing or over-dosing. There is a reasonable probability that under-dosing may result in lower exposures and decrease in efficacy which could ultimately lead to graft rejection and graft loss in transplant patients. Furthermore, over-dosage may manifest itself as cyclosporine toxicity in the long term if over exposure continues. Novartis has not received any reports of adverse events related to this recall, to date.
The affected lot number and expiration date is: FX001691 (expiration date 12/2025). This lot was distributed nationwide to wholesalers across the US, beginning in April 2023.
Novartis is notifying its distributors via a recall notification letter and is arranging for return of the recalled lot from distributors, retailers and consumers. Additionally, Novartis is notifying health care providers who have prescribed this product to contact their patients. Consumers that have bottles from the recalled lot of Sandimmune Oral Solution (cyclosporine oral solution, USP), 100mg/mL, should contact their health care provider.
In the event that a patient experiences an adverse reaction or quality problem involving this product, they should immediately contact their health care provider and Novartis to report the event or finding. Patients or health care providers may call the Novartis customer interaction center at 888-NOW-NOVA (888-669-6682) from 8:30 AM – 5:00 PM ET Monday through Friday or may report an adverse event through https://www.novartis.com/report or usdrugsafety.operations@novartis.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “may,” “could,”“would,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this press release, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding such products could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures and requirements for increased pricing transparency; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political, economic and business conditions, including the effects of and efforts to mitigate pandemic diseases such as COVID-19; safety, quality, data integrity or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.
About Novartis
Novartis is reimagining medicine to improve and extend people’s lives. We deliver high-value medicines that alleviate society’s greatest disease burdens through technology leadership in R&D and novel access approaches. In our quest to find new medicines, we consistently rank among the world’s top companies investing in research and development. Novartis employs about 14,000 people in the United States. For more information, please visit https://www.novartis.us
Novartis and Novartis US is on Twitter. Sign up to follow @Novartis at https://twitter.com/novartisnews and @NovartisUS at https://twitter.com/NovartisUS. For Novartis multimedia content, please visit https://www.novartis.com/news/medialibrary.
For questions about the site or required registration, please contact media.relations@novartis.com.
References
1. Sandimmune [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021.
# # #
Novartis Media Relations
E-mail: media.relations@novartis.com
North America
Julie Masow
+1 862 579 8456
Michael Meo
+1 862 274 5414
Marlena Abdinoor
+1 617 335 9525
Novartis Investor Relations
E-mail: investor.relations@novartis.com
North America
Sloan Simpson +1 862 778 5052
Company Contact Information
- Consumers:
- Novartis Pharmaceuticals Corporation
- 1-888-669-6682
- Media:
- Novartis Media Relations – North America – Julie Masow, Michael Meo, Marlena Abdinoor
- 1-862-579-8456, 1-862-274-5414, 1-617-335-9525
- media.relations@novartis.com
Leave a Reply