Summary
- Company Announcement Date:
- October 19, 2021
- FDA Publish Date:
- October 20, 2021
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Product contains particulate matter identified as glass
- Company Name:
- Merck
- Brand Name:
-
Brand Name(s)
- Product Description:
-
Product Description
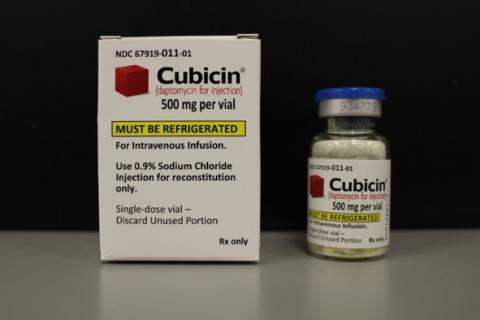
Daptomycin for injection 500mg
Company Announcement
KENILWORTH, N.J., Oct. 19, 2021 – Merck (NYSE: MRK), known as MSD outside the United States and Canada, is voluntarily recalling one lot of CUBICIN® (daptomycin for injection) 500mg for intravenous use, Lot 934778, Exp June 2022. Because treatment with CUBICIN is likely to be initiated at a hospital or other monitored healthcare setting, the recall is being conducted to the user level, including hospital and administering institutions. Approximately 22,000 vials are affected by this recall. The recall has been initiated following receipt of a customer complaint reporting that a piece of glass was found in a vial of CUBICIN after reconstitution.
Intravenous infusion of glass particulates has the potential to cause serious health consequences if the particulate is small enough to be withdrawn from the vial and infused into the patient. Local irritation or swelling at the infusion site may occur in response to the presence of foreign material. More serious potential outcomes include blockage and clotting in blood vessels, which can be life-threatening if a critical organ is affected. Other clinical consequences could include prolonged hospitalization, particularly in those patients receiving an extended treatment regimen for which multiple vials of CUBICIN are administered over the course of treatment. The risk is reduced by the possibility of detection, as the label contains a clear statement directing the healthcare professional to visually inspect the product for particulate matter prior to administration. To date, Merck has not received any reports of adverse events related to this recall.
CUBICIN is a lipopeptide antibacterial indicated for the treatment of complicated skin and skin structure infections (cSSSI) in adult and pediatric patients (1 to 17 years of age), and Staphylococcus aureus bloodstream infections (bacteremia) in adult patients including those with right-sided infective endocarditis, and Staphylococcus aureus bloodstream infections (bacteremia) in pediatric patients (1 to 17 years of age). CUBICIN is packaged in single-dose, 10 mL glass vial containing 500 mg of daptomycin as a sterile, lyophilized cake, NDC 67919-011-01. There were 76,163 total vials manufactured in the affected lot; and of those vials, 21,603 have been distributed. The affected CUBICIN lot includes the following: Bulk drug product and packaged lot 934778, expiration date June 2022. This product was distributed to wholesalers between June 1, 2021, and Sept. 9, 2021, in the U.S.
Merck is notifying its distributors and customers and is arranging for return of all recalled product by sending recall notices via UPS Next Day Air to all nine Merck direct consignees with a physical address. Recall notices are being sent via USPS to the Merck direct consignees with a US Post Office address. All Merck direct consignees will be instructed to perform a sub-recall and notify all customers to whom they had distributed the lot.
Customers that have CUBICIN vials from the lot that is being recalled should contact Sedgwick at (877) 830-9730 for product return instructions. Sedgwick will provide all customers with prepaid shipping labels, packing slips and business reply cards.
Consumers with questions regarding this recall can contact the Merck National Service Center at (800) 672-6372, select prompt #1, then please hold for a representative. (Monday to Friday 8:00 AM to 7:00 PM (EST). Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
“Our highest priorities are the health and safety of patients and the quality of our medicines and vaccines,” said Richard M. Haupt, M.D., MPH, Vice President and Head, Vaccines & Infectious Diseases, Merck Research Labs. “We sincerely regret any inconvenience caused by this recall.”
About Merck
For over 130 years, Merck, known as MSD outside of the United States and Canada, has been inventing for life, bringing forward medicines and vaccines for many of the world’s most challenging diseases in pursuit of our mission to save and improve lives. We demonstrate our commitment to patients and population health by increasing access to health care through farreaching policies, programs and partnerships. Today, Merck continues to be at the forefront of research to prevent and treat diseases that threaten people and animals – including cancer, infectious diseases such as HIV and Ebola, and emerging animal diseases – as we aspire to be the premier research-intensive biopharmaceutical company in the world. For more information, visit www.merck.comand connect with us on Twitter,Facebook,Instagram,YouTubeand LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Kenilworth, N.J., USA
This news release of Merck & Co., Inc., Kenilworth, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s 2020 Annual Report on Form 10-K and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).
Company Contact Information
- Consumers:
- Merck National Service
- (800) 672-6372
- Media:
- Patrick Ryan, Melissa Moody
- (973) 275-7075, (215) 407-3536
Leave a Reply