Summary
- Company Announcement Date:
- March 04, 2020
- FDA Publish Date:
- March 05, 2020
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Potential Presence of Small Particulates
- Company Name:
- Hikma Pharmaceuticals USA Inc.
- Brand Name:
-
Brand Name(s)
- Product Description:
-
Product Description
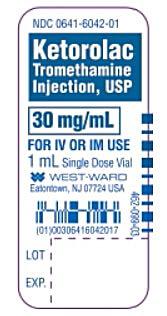
Ketorolac Tromethamine Injection, USP 30mg/mL, 1 mL Vial
Company Announcement
Hikma Pharmaceuticals PLC (Hikma, Group), today announces that its subsidiary Hikma Pharmaceuticals USA Inc. (formerly known as West-Ward Pharmaceuticals Corp.; “Hikma”) is voluntarily extending its previously-announced recall of certain lots (listed below) of Ketorolac Tromethamine Injection USP 30mg/mL, 1mL fill/2mL vials to the medical facility and retail levels. The product is being recalled due to the presence of small visible particulate matters of a gelatinous/oily nature that appear black in some of the recalled lots.
On December 23, 2019, Hikma voluntarily initiated a recall of this product to the direct customer level. In coordination with the U.S. Food and Drug Administration (FDA), Hikma is extending the recall to the medical facility and retail levels.
Administration of the affected product could potentially result in the deposition of particulates in the lungs of patients, which could result in multiple pulmonary microemboli with subsequent acute respiratory distress for patients receiving the drug intravenously.
Although Hikma has not received any reports of adverse events related to this issue, it is nonetheless extending its recall of these products out of an abundance of caution and to promote patient safety, which is Hikma’s highest priority.
The lots being recalled were manufactured between March 22, 2018, and February 21, 2019. Hikma investigated the cause of the problem and decided to suspend manufacturing of this product until an appropriate solution can be implemented to prevent recurrence.
Ketorolac Tromethamine Injection is a nonsteroidal anti-inflammatory drug (“NSAID”) that is indicated for the short-term (up to 5 days in adults) management of moderately severe acute pain that requires analgesia at the opioid level.
The affected lot numbers and expiration dates being recalled are as follows:
| NDC | Product Name and Strength | Size | Lot Number | Expiration Date |
|---|---|---|---|---|
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 038366 | Mar-2020 |
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 048365 | Apr-2020 |
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 048367 | Apr-2020 |
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 078301 | Jul-2020 |
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 078303 | Jul-2020 |
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 118358 | Nov-2020 |
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 019413 | Jan-2021 |
| 0641-6042-25 | Ketorolac Tromethamine Injection 30mg/mL | 1mL Fill/2mL Vial | 029353 | Feb-2021 |
The product can be identified by name and NDC and lot codes, which are clearly stated on the product label, along with Hikma Pharmaceuticals USA Inc./West-Ward Pharmaceutical Corp.’s name and address. Images of the vial and shelf pack labels are included below:
The product was distributed to Hikma’s direct customers nationwide. Hikma notified its direct customers as part of the initial recall, asking them to contact medical and retail level facilities to locate and remove the recalled product from distribution channels and return the recalled lots to Hikma. Hikma is now asking customers at the medical and retail level facilities to locate and remove the recalled product from their channels and return the recalled lots to Hikma.
For recall inquiries, please contact Hikma using the information provided below:
| Hikma Contact | Contact Information | Areas of Support |
|---|---|---|
| Hikma Pharmaceuticals USA Inc. – Customer Service | (P): (800) 631-2174 (E): usrecall@hikma.com (F): (732) 542-0940 Hours of operation, M-F: 8:00am-7pm (EST) |
Recall related inquires |
| Qualanex LLC (Hikma 3rd Party Recall Service Provider) | Qualanex LLC 1410 Harris Road Libertyville, IL 60048-2435 (E): recall@qualanex.com |
Product Returns |
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Enquiries:
Steve Weiss +1 732 720 2830/ +1 732 788 8279
David Belian +1 732 720 2814/ +1 848 254 4875
US Communications and Public Affairs uscommunications@hikma.com
About Hikma
Hikma helps put better health within reach every day for millions of people in more than 50 countries around the world. For more than 40 years, we’ve been creating high-quality medicines and making them accessible to the people who need them. Headquartered in the UK, we are a global company with a local presence across the United States (US), the Middle East and North Africa (MENA) and Europe, and we use our unique insight and expertise to transform cutting-edge science into innovative solutions that transform people’s lives. We’re committed to our customers, and the people they care for, and by thinking creatively and acting practically, we provide them with a broad range of branded and non-branded generic medicines. Together, our 8,600 colleagues are helping to shape a healthier world that enriches all our communities. We are a leading licensing partner, and through our venture capital arm, are helping bring innovative health technologies to people around the world. For more information, please visit: www.hikma.com

Leave a Reply