Summary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Potential presence of N-Nitrosodimethylamine (NDMA) above levels established by the FDA
- Company Name:
- GSMS, Inc.
- Brand Name:
-
Brand Name(s)
GSMS Incorporated
- Product Description:
-
Product Description
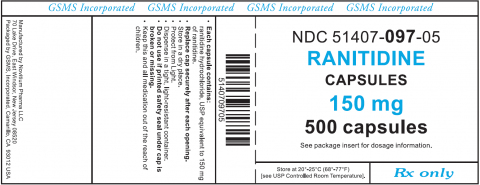
Ranitidine HCl 150mg and 300mg Capsules
Company Announcement
Golden State Medical Supply, Incorporated (GSMS, Inc) based upon a manufacturer’s recall by Novitium Pharma LLC (Novitium), is voluntarily instituting a recall of all quantities and lots, within expiry, of Ranitidine HCl 150mg and 300mg Capsules to the consumer level because of potential presence of N-Nitrosodimethylamine (NDMA) above levels established by the FDA.
GSMS, Inc. received a recall notification from the manufacturer, Novitium, on October 25th, 2019 and initiated a voluntary recall on November 6th, 2019 to the consumer level for 7 lots of Ranitidine HCl 150mg and 12 lots of Ranitidine HCl 300mg Capsules.
(Novitium’s press release: https://novitiumpharma.com/products/ranitidine-recall-press-release-final/)
To date, GSMS, Inc. has not received any reports of adverse events related to the use of the products as part of this recall.
Risk Statement: NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.
Ranitidine Hydrochloride Capsules are indicated for the treatment of duodenal ulcer, benign gastric ulcer, reflux esophagitis, post-operative peptic ulcer, Zollinger-Ellison Syndrome, and other conditions where reduction of gastric secretion and acid output is desirable.
The Ranitidine Hydrochloride Capsules being recalled are supplied as follows:
- Ranitidine Hydrochloride 150mg Capsules: Caramel colored capsules, imprinted 001 on cap and novitium 150 mg on body in white ink, filled with white to pale yellow powder.
- Ranitidine Hydrochloride 300mg Capsules: Caramel colored capsules, imprinted 002 on cap and novitium 300 mg on body in white ink, filled with white to pale yellow powder.
GSMS, Inc. has promptly notified its consignees via email and certified mail. Consignees, i.e., wholesale distributors and TMOPs, are instructed to immediately stop distribution, quarantine all remaining products in their control, and return the recalled product to GSMS, Inc. The consignees are also instructed to provide their customers, i.e., pharmacies and consumers, a copy of GSMS, Inc.’s recall notification, recall response form, and letter to consumers, patients, and caregivers.
The lots under GSMS, Inc.’s voluntary recall have been primarily sold to AmerisourceBergen, McKesson, and Tricare Mail Order Pharmacies (TMOP) as listed below:
| Product Description | GSMS NDC | GSMS Lot # | Expiration Date |
|---|---|---|---|
| RANITIDINE HCl 150MG CAPSULES 500 Ct Bottle | 51407-097-05 | GS023970 | 10/31/2020 |
| GS026108 | 10/31/2020 | ||
| GS026099 | 10/31/2020 | ||
| GS026838 | 10/31/2020 | ||
| GS025702 | 10/31/2020 | ||
| GS027272 | 10/31/2020 | ||
| GS027273 | 05/31/2021 | ||
| RANITIDINE HCl 300MG CAPSULES 100 Ct Bottle | 51407-098-01 | GS023971 | 10/31/2020 |
| GS025527 | 10/31/2020 | ||
| GS025526 | 10/31/2020 | ||
| GS026114 | 10/31/2020 | ||
| GS025813 | 10/31/2020 | ||
| GS026189 | 10/31/2020 | ||
| GS027555 | 07/31/2021 | ||
| GS026190 | 05/31/2021 | ||
| GS026220 | 05/31/2021 | ||
| GS026584 | 05/31/2021 | ||
| GS027139 | 05/31/2021 | ||
| GS027554 | 05/31/2021 |
Consumers taking Ranitidine HCl capsules are advised to consult with their physician, pharmacist, or healthcare provider about an alternative treatment prior to discontinuing the use of the medication. Once an alternative treatment has been prescribed, the patients are instructed to contact Novitium‘s recall processor to obtain instructions and kit for returning the remaining product in their possession.
Manufacturer: Novitium Pharma
Recall Processor: Stericycle Return Management Services
Phone: (866) 382-8606
Email: Novitiumpharma6639@stericycle.com
Consumers with questions regarding this recall can contact GSMS, Inc, by:
Phone: (800) 284-8633, ext. 215 (Monday through Friday between 7:30 AM to 4:00 PM, Pacific)
Fax: (805) 437-7588
Email: recalls@gsms.us
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Media:
- Tammy Stefanovic
- (805) 477-9866 ext. 287

Leave a Reply