Summary Company Announcement Date: December 27, 2024 FDA Publish Date: December 27, 2024 Product Type: Food & Beverages … [Read more...]
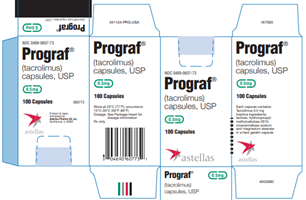
Astellas Pharma US, Inc. Issues Voluntary Nationwide Recall of One Lot of PROGRAF® 0.5mg (Tacrolimus) and One Lot of ASTAGRAF XL® 0.5mg (Tacrolimus Extended-Release Capsules) Because Bottles Shipped to U.S. May Contain Empty Capsules
Summary Company Announcement Date: December 23, 2024 FDA Publish Date: December 24, 2024 Product Type: Drugs … [Read more...]
Alcon Laboratories Issues Voluntary Nationwide Recall of One (1) Lot of Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, 25 count (Lot 10101), Due to Fungal Contamination
Summary Company Announcement Date: December 21, 2024 FDA Publish Date: December 23, 2024 Product Type: Drugs … [Read more...]
Lidl Recalls Taste of Deutschland Buttered Vegetables Due to Undeclared Milk Allergens
Summary Company Announcement Date: December 20, 2024 FDA Publish Date: December 20, 2024 Product Type: Food & Beverages … [Read more...]
Endo USA, Inc. Issues Voluntary, Nationwide Recall of Adrenalin® Chloride Solution (EPINEPHrine Nasal Solution, USP) Due to the Potential for Administration Errors
Summary Company Announcement Date: December 20, 2024 FDA Publish Date: December 20, 2024 Product Type: Drugs … [Read more...]
- « Previous Page
- 1
- …
- 32
- 33
- 34
- 35
- 36
- …
- 1297
- Next Page »



Recent Comments