Summary Company Announcement Date: October 19, 2021 FDA Publish Date: October 20, 2021 Product Type: Drugs … [Read more...]
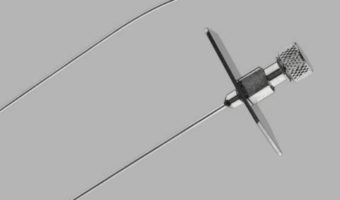
Cook Medical Issues Voluntary Recall of Transseptal Needle and Transseptal Needle with Catheter
Summary Company Announcement Date: October 18, 2021 FDA Publish Date: October 19, 2021 Product Type: Medical Devices … [Read more...]
Bryant Ranch Prepack Issues Voluntary Nationwide Recall of Methocarbamol 500mg Bottles Due to Mislabeling With the Incorrect Strength
Summary Company Announcement Date: October 18, 2021 FDA Publish Date: October 19, 2021 Product Type: Drugs … [Read more...]
Lupin Pharmaceuticals, Inc. Issues Voluntarily Nationwide Recall of All Irbesartan Tablets and Irbesartan and Hydrochlorothiazide Tablets Due to Potential Presence of N-nitrosoirbesartan Impurity
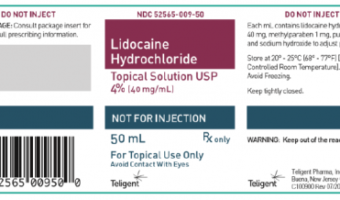
Summary Company Announcement Date: October 14, 2021 FDA Publish Date: October 14, 2021 Product Type: Drugs … [Read more...]
Teligent Pharma, Inc.’s Issues Worldwide Voluntary Recall of Lidocaine HCl Topical Solution 4% Due to Super Potency
Summary Company Announcement Date: October 12, 2021 FDA Publish Date: October 12, 2021 Product Type: Drugs … [Read more...]
- « Previous Page
- 1
- …
- 235
- 236
- 237
- 238
- 239
- …
- 1297
- Next Page »

Recent Comments