Summary Company Announcement Date: November 21, 2019 FDA Publish Date: November 21, 2019 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Undeclared sulfites Company Name: Euroline Foods LLC Brand Name: Brand Name(s) Tainy Vostoka Product Description: Product Description Dried Fruit … [Read more...]
Mondelēz Global LLC Conducts Voluntary Recall of Limited Quantity of Cheese Nips (11 oz. Box) Product in the U.S. Due to Potential Presence of Foreign Material
Summary Company Announcement Date: November 15, 2019 FDA Publish Date: November 20, 2019 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Potential presence of plastic Company Name: Mondelēz Global LLC Brand Name: Brand Name(s) Product Description: Product Description Cheese Nips Company … [Read more...]
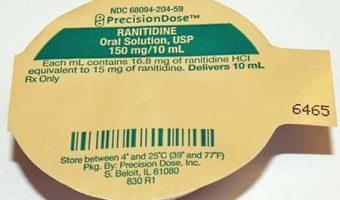
Precision Dose Inc. Issues Voluntary Nationwide Recall of Ranitidine Oral Solution, USP 150 mg/10 mL Due to Possible Presence of N-nitrosodimethylamine (NDMA) Impurity.
Summary Company Announcement Date: November 19, 2019 FDA Publish Date: November 19, 2019 Product Type: Drugs Reason for Announcement: Recall Reason Description Potential presence of N-Nitrosodimethylamine (NDMA) above levels established by the FDA Company Name: Precision Dose Inc. Brand Name: Brand Name(s) PrecisionDose Product … [Read more...]
Select Varieties of Breakstone’s Cottage Cheese are Voluntarily Being Recalled Due to Potential Presence of Foreign Material
Summary Company Announcement Date: November 15, 2019 FDA Publish Date: November 15, 2019 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Potential presence of plastic and metal Company Name: Kraft Heinz Foods Company Brand Name: Brand Name(s) Breakstone’s Product Description: Product … [Read more...]
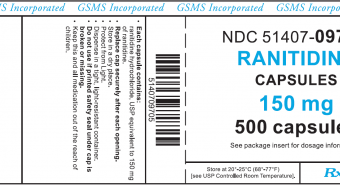
Golden State Medical Supply, Inc. Issues a Voluntary Nationwide Recall of Ranitidine Hydrochloride 150mg and 300mg Capsules (Manufactured by Novitium Pharma LLC) Due to an Elevated Amount of Unexpected Impurity, N-Nitrosodimethylamine (NDMA)
Summary Company Announcement Date: November 15, 2019 FDA Publish Date: November 15, 2019 Product Type: Drugs Reason for Announcement: Recall Reason Description Potential presence of N-Nitrosodimethylamine (NDMA) above levels established by the FDA Company Name: GSMS, Inc. Brand Name: Brand Name(s) GSMS Incorporated Product … [Read more...]
- « Previous Page
- 1
- …
- 356
- 357
- 358
- 359
- 360
- …
- 816
- Next Page »



Recent Comments