Summary Company Announcement Date: February 07, 2020 FDA Publish Date: February 07, 2020 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Potential contamination with Listeria sp, Company Name: Nuts ‘N More LLC Brand Name: Brand Name(s) Nuts ‘N More LLC Product Description: Product … [Read more...]
New Seasons Market Recalls Grab N’ Go Vegetarian Lasagna w/Gluten Free Pasta Due to Undeclared Soy
Summary Company Announcement Date: February 06, 2020 FDA Publish Date: February 06, 2020 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Undeclared Soy Company Name: New Seasons Market Brand Name: Brand Name(s) New Seasons Market Product Description: Product Description Grab N’ Go Vegetarian … [Read more...]
Cargill Conducts Voluntary Recall of a Single Lot of Nutrebeef® Transition Pellet Due to Elevated Levels of Monesin
Cargill’s animal nutrition business is conducting a voluntary recall of a single lot (382 bags, 50 lb each) of NutreBeef® Transition Pellet (MH) beef cattle feed because the product may contain mispackaged feed with elevated levels of monensin … [Read more...]
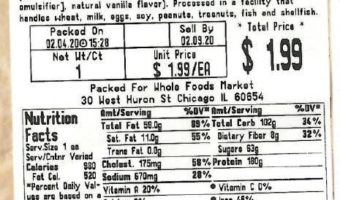
Allergy Alert Issued for Undeclared Milk in Bundt Cake and Chocolate Dipped Cookies Sold at 44 Whole Foods Market Stores in the Mid-Atlantic Region
Summary Company Announcement Date: February 04, 2020 FDA Publish Date: February 04, 2020 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Undeclared milk Company Name: Whole Foods Market Brand Name: Brand Name(s) Whole Foods Market Product Description: Product Description Bundt Cake and Chocolate … [Read more...]
Efficient Laboratories, Inc. Issues Voluntary Nationwide Recall of Rompe Pecho EX, Rompe Pecho CF, and Rompe Pecho MAX due to Microbial Contamination
Summary Company Announcement Date: January 31, 2020 FDA Publish Date: January 31, 2020 Product Type: Drugs Reason for Announcement: Recall Reason Description due to Microbial Contamination Company Name: Efficient Laboratories, Inc. Brand Name: Brand Name(s) Product Description: Product Description Rompe Pecho EX, Rompe Pecho CF, and … [Read more...]
- « Previous Page
- 1
- …
- 343
- 344
- 345
- 346
- 347
- …
- 816
- Next Page »




Recent Comments