Summary
- Company Announcement Date:
- June 02, 2025
- FDA Publish Date:
- June 04, 2025
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason Description
Microbial contamination
- Company Name:
- Amneal Pharmaceutical LLC
- Brand Name:
-
Brand Name(s)
- Product Description:
-
Product Description
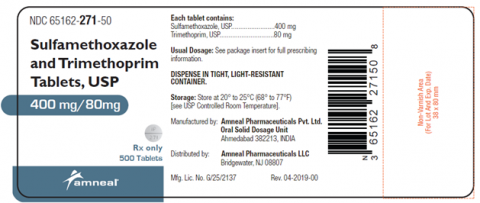
Sulfamethoxazole/Trimethoprim Tablets, USP, 400 mg/80 mg
Company Announcement
Amneal Pharmaceutical LLC, is recalling three lots of Sulfamethoxazole/Trimethoprim Tablets, USP, 400 mg/80 mg to the consumer level as the tablets may exhibit black spots on the tablet surface due to microbial contamination. The observance of black spots was reported in a product quality complaint.
Risk Statement: Oral products contaminated with Aspergillus may result in serious and life-threatening infections. The use of the defective product in patients with underlying immunosuppressive conditions increases the concern for serious infections. To date, Amneal Pharmaceuticals has received no reports of adverse events, illnesses or injuries related to this recall.
The recalled product was distributed nationwide to wholesalers/distributors between the dates of 12/4/2024 to 5/15/2025 only.
The product is indicated for the treatment of Urinary tract infections caused by susceptible strains of the following organisms: Escherichia Coli, Klebsiella species, Enterobacter species, Morganella morganii, Proteus mirabilis and Proteus vulgaris. Acute otitis media in pediatric patients. Acute exacerbations of chronic bronchitis due to susceptible strains of Streptococcus pneumoniae. Enteritis caused by susceptible strains of Shigella flexneri and traveler’s diarrhea in adults.
Sulfamethoxazole/Trimethoprim Tablets, USP, 400 mg/80 mg is packaged in 100 tablet count and 500 tablet count bottles. This recall pertains only to the 400 mg/80 mg strength and only to the listed Lots below. The lot number can be found on the Amneal bottle label or consult your pharmacy if you received a pharmacy vial. No other Sulfamethoxazole/Trimethoprim Tablets, USP, 400 mg/80 mg lots are impacted.
| Lot Number | NDC Number | Expiration date | Date of First Distribution | Bottle Pack Size |
|---|---|---|---|---|
| AM241019 | 65162-271-10 | 06/2027 | 12/4/2024 | 100 count |
| AM241019A | 65162-271-50 | 06/2027 | 12/4/2024 | 500 count |
| AM241020 | 65162-271-10 | 06/2027 | 12/4/2024 | 100 count |
Amneal is notifying its customers by UPS and is arranging for return of all recalled products. Wholesalers/distributors are being asked to notify their customers of the recall and provide instructions to contact Amneal for the return of the recalled products to Amneal. Retailers are being asked to notify their customers and instruct consumers to contact Amneal directly for assistance with return of any recalled product and reimbursement information.
Individuals with questions regarding this recall can contact Amneal Pharmaceuticals by:
For Medical Inquiries or to report Adverse Events, or quality problems experienced with the use of this product, please contact Amneal Drug Safety by phone at 1-877-835-5472, Monday – Friday, 8:00 am – 6:00 pm, EST, or e-mail at DrugSafety@amneal.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
Product Photos






Leave a Reply