Summary Company Announcement Date: June 12, 2025 FDA Publish Date: June 12, 2025 Product Type: Food & Beverages … [Read more...]
Search Results for: labeling
T.W. Garner Food Company Issues Recall on Texas Pete® Habanero Buffalo Sauce Due To Potential Presence of Undeclared Sulfites and Sweet CHAbanero Sweet Sriracha Habanero Sauce Due To Mislabeling
Summary Company Announcement Date: April 03, 2025 FDA Publish Date: April 03, 2025 Product Type: Food & Beverages … [Read more...]
Dr. Reddy’s Issues a Nationwide Recall of Levetiracetam in 0.75% Sodium Chloride Injection 1,000 mg/100 mL, in the U.S., Due to Mislabeling of Infusion Bag
Summary Company Announcement Date: March 13, 2025 FDA Publish Date: March 13, 2025 Product Type: Drugs … [Read more...]
ICU Medical Issues Nationwide Recall of Potassium Chloride Injection, 20 mEq and Potassium Chloride Injection, 10 mEq Due to Mislabeling
Summary Company Announcement Date: February 13, 2025 FDA Publish Date: February 14, 2025 Product Type: Drugs … [Read more...]
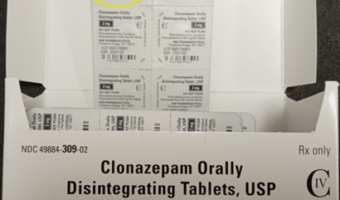
Endo Expands Voluntary Recall of Clonazepam Orally Disintegrating Tablets, USP (C-IV) Due to Potential Product Carton Strength Mislabeling
Summary Company Announcement Date: November 18, 2024 FDA Publish Date: November 19, 2024 Product Type: Drugs … [Read more...]
- 1
- 2
- 3
- …
- 112
- Next Page »

Recent Comments