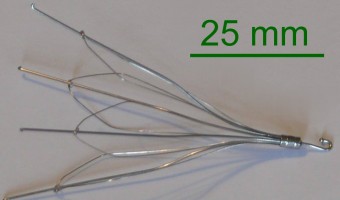
A retrievable inferior vena cava (IVC) filter is a medical device used as an alternative to anticoagulant drugs in people who are at risk for pulmonary embolism (PE) but cannot use the drugs or for whom the drugs are ineffective. There are several ways in which an IVC filter can fail, including migration of the device or broken pieces of the device … [Read more...]
Three Legal Paths for IVC Filter Litigants to Explore
There have been significant developments in the potential for IVC Filter litigation. The safety of IVC Filters was first highlighted by the FDA in August 2010, with over 900 problem cases being reported. Since then, the FDA has carried out further investigations into the issues of IVC Filters moving, failing entirely or having parts come away. In … [Read more...]
Exploring the IVC Filter Decision Analysis Research
The FDA has been drawn into a deeper analysis of the medical impact of the failings that have been highlighted in several brands of IVC Filters. These devices are intended to treat blood clots, but have been found to fail more often then would be considered normal, let alone acceptable. In 2010 the FDA issued a safety alert highlighting the … [Read more...]
FDA Updates on the Safety of IVC Filters
In August 2010 the FDA issued a safety warning about the use of IVC Filters, which are commonly used in the treatment of deep-vein thrombosis. This arose following reports since 2005 to the Food and Drug Administration of issues regarding the use of these filters and was elevated to the status of 'Safety Alert' following 921 adverse reports into … [Read more...]
Are IVC Filters Safe?
IVC Filters have been in the news recently and for all the wrong reasons. The New Jersey firm of C.R. Bard, Inc. has been put in the NBC Nightly News spotlight after NBC’s investigation linked C.R. Bard’s brand of IVC Filters to 300 injuries and 27 deaths. The most common reason for using IVC filters is to stop blood clots from reaching key … [Read more...]


Recent Comments