Summary
- Company Announcement Date:
- May 11, 2019
- FDA Publish Date:
- May 12, 2019
- Product Type:
- Drugs
Generic Drugs - Reason for Announcement:
-
Recall Reason
Device & Drug Safety, Potential Foreign Material
- Company Name:
- Novartis
- Brand Name:
-
Brand Name(s)
- Product Description:
-
Product Description
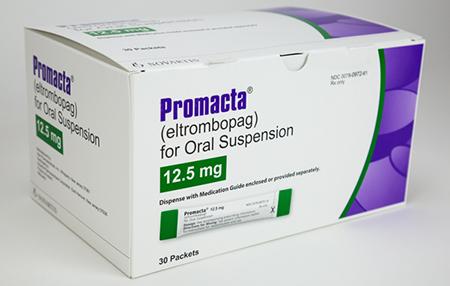
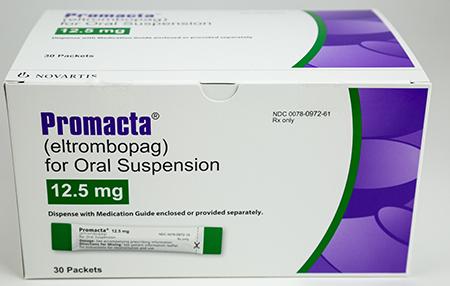
Promacta 12.5 mg oral suspension
Company Announcement
Novartis today announced a voluntary recall of three lots of Promacta (eltrombopag) 12.5 mg for oral suspension to the consumer level. The oral suspension lots are being recalled because of a risk of potential peanut flour contamination that occurred at a third-party contract manufacturing site.
Promacta tablets in 12.5 mg, 25 mg, 50 mg and 75 mg strengths are not impacted by this recall and are not manufactured in the same facility.
Peanut is a known food allergen. Potential cross contamination with peanut flour, even in small traces, can lead to hypersensitivity reaction in a population of patients with an unknown or known sensitivity to peanut antigen, including a medically significant anaphylactic reaction, which can be fatal.
To date, Novartis has not received any reports or adverse events for this recall.
Promacta 12.5 mg for oral suspension is indicated for the treatment of certain adult and pediatric patients with chronic immune thrombocytopenia, certain adult patients with hepatitis C-associated thrombocytopenia, and certain adult and pediatric patients with severe aplastic anemia who have not received prior immunosuppressive therapy or had an insufficient response to immunosuppressive therapy. See promacta.com for full prescribing information.
Promacta 12.5 mg for oral suspension was distributed nationwide through specialty pharmacies. Novartis is notifying its distributors and customers by letter and asking them to check for impacted product and to return unused product through directions provided in the recall letter. The affected product name, including the lot numbers and expiration dates, include:
Impacted Promacta 12.5 mg for Oral Suspension Lot Numbers:
| Product Description | NDC Number on Carton | NDC Number on Packet | Lot Number | Expiration Date | Distribution Dates |
|---|---|---|---|---|---|
| Promacta for Oral Suspension | 0078-0972-61 | 0078-0972-19 | 8H57901589 | 09/2020 | 1/2/19 – 2/11/19 |
| Promacta for Oral Suspension | 0078-0972-61 | 0078-0972-19 | 9H57900189 | 12/2020 | 2/11/19 – 4/17/19 |
| Promacta for Oral Suspension | 0078-0972-61 | 0078-0972-19 | 9H57900289 | 12/2020 | 3/6/19 – 4/2/19 |
Consumers who have impacted product with these lot numbers and NDC numbers in their homes should contact 1-866-918-8772 (8:00 AM – 5:00 PM EST, Monday through Friday) for instructions on how to return recalled product. For all additional questions, please contact Novartis at 1-888-NOW NOVA (8:30 AM – 5:00 PM EST, Monday through Friday).
Consumers should stop taking Promacta 12.5 mg oral suspension and consult with their healthcare provider. Consumers should also contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Pharmacies that have impacted product with these lot numbers and NDC numbers should contact 1-866-918-8772 (8:00 AM – 5:00 PM EST, Monday through Friday) for instructions for return of recalled product.
Healthcare professionals with questions can contact Novartis Medical Information at 1-844-ONC-INFO (1-844-622-4636) or at USOncology.MedInfo@novartis.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding the voluntary recall of three lots of Promacta 12.5 mg for oral suspension, potential marketing approvals, new indications or labeling for the investigational or approved products described in this press release, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that additional lots of Promacta 12.5 mg for oral suspension will not be recalled. Neither can there be any guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding Promacta for oral suspension and such other products could be affected by, among other things, safety, quality or manufacturing issues; the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political and economic conditions; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.
About Novartis
Novartis is reimagining medicine to improve and extend people’s lives. As a leading global medicines company, we use innovative science and digital technologies to create transformative treatments in areas of great medical need. In our quest to find new medicines, we consistently rank among the world’s top companies investing in research and development. Novartis products reach more than 750 million people globally and we are finding innovative ways to expand access to our latest treatments. About 105 000 people of nearly 140 nationalities work at Novartis around the world. Novartis Pharmaceuticals Corporation, a US affiliate of Novartis, is located in East Hanover, NJ. Find out more at www.novartis.com.
Novartis is on Twitter. Sign up to follow @Novartis at https://twitter.com/novartis
For Novartis multimedia content, please visit www.novartis.com/news/media-library
For questions about the site or required registration, please contact media.relations@novartis.com
Novartis Media Relations
Central media line: +41 61 324 2200
E-mail: media.relations@novartis.com
Novartis Investor Relations
Central investor relations line: +41 61 324 7944
E-mail: investor.relations@novartis.com
Central North America
Samir Shah +41 61 324 7944 Richard Pulik +1 212 830 2448
Pierre-Michel Bringer +41 61 324 1065 Cory Twining +1 212 830 2417
Thomas Hungerbuehler +41 61 324 8425
Isabella Zinck +41 61 324 7188
Company Contact Information
- Consumers:
- 1-866-918-8772
Leave a Reply