Summary
- Company Announcement Date:
- May 04, 2021
- FDA Publish Date:
- May 04, 2021
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Due to mislabeling
- Company Name:
- Hospira, Inc.
- Brand Name:
-
Brand Name(s)
Hospira, Inc.
- Product Description:
-
Product Description
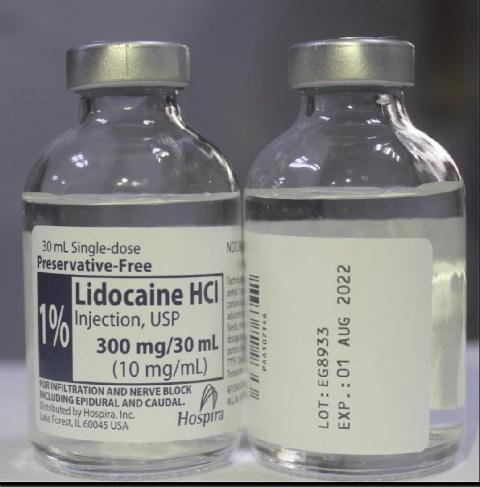
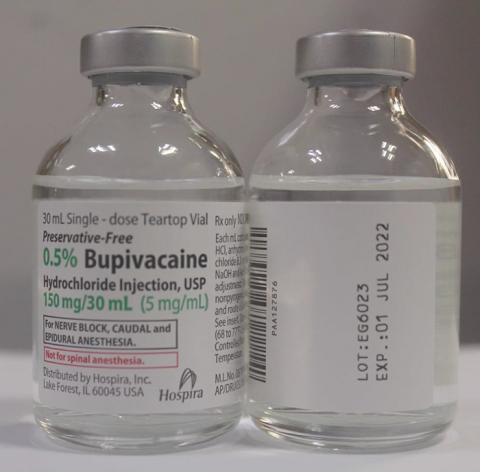
0.5% Bupivacaine Hydrochloride Injection, USP 30 mL and 1% Lidocaine HCl Injection, USP 30 mL
Company Announcement
Hospira, Inc., a Pfizer company, is voluntarily recalling lot EG6023 of 0.5% Bupivacaine Hydrochloride Injection, USP 30 mL and lot EG8933 of 1% Lidocaine HCl Injection, USP 30 mL, to the hospital/institution level due to mislabeling whereby a portion of each lot was incorrectly labeled as the other product. This issue was identified as part of the investigation of a confirmed customer report.
Hospira’s assessment of the potential risk to patients concluded that the use of the impacted product is likely to cause adverse events of moderate to high severity. If 1% lidocaine is administered to the patient instead of 0.5% bupivacaine, the patient may be underdosed, leading to lack of efficacy with potential outcomes such as inadequate pain management, and failure of surgical anesthesia. If 0.5% bupivacaine is administered to the patient instead of 1% lidocaine, an overdose of bupivacaine may occur, which could lead to potential outcomes such as seizures; respiratory abnormalities including low oxygen and/or elevated carbon dioxide in the blood, too much acid in the body fluids, and temporary cessation of breathing; heart abnormalities such as heart contraction and/or relaxation issues, irregular heartbeat, slower than normal heart rate, abnormal heart rhythm in which the ventricles of the heart quiver instead of pumping normally, cardiac arrest and cardiac flatline. To date, Hospira, Inc. has not received reports of any adverse events associated with this issue for these lots.
0.5% Bupivacaine Hydrochloride Injection, USP is indicated in adults for the production of local or regional anesthesia or analgesia for surgery, dental and oral surgery procedures, diagnostic and therapeutic procedures, and obstetrical procedures.
1% Lidocaine HCl Injection, USP is indicated for the production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous regional anesthesia by peripheral nerve block techniques such as brachial plexus and intercostal and by central neural techniques such as lumbar and caudal epidural blocks when the accepted procedures for these techniques as described in standard textbooks are observed.
The NDC, Lot Number, Expiration Date, Strength and Configuration details for 0.5% Bupivacaine Hydrochloride Injection, USP and 1% Lidocaine HCl Injection, USP are in the table below and a photo of the products can be found at the end of this press release. The product lots were distributed nationwide to wholesalers, distributors, retailers, and hospitals in the United States, Puerto Rico and Guam from December 29, 2020 to April 15, 2021.
| Product | NDC | Lot Number | Expiration Date | Strength | Configuration/ Count |
|---|---|---|---|---|---|
| 0.5% Bupivacaine Hydrochloride Injection, USP, Single Dose Teartop Vial |
Vial: 0409-1162-19 Tray: 0409-1162-02 |
EG6023 | 01 July 2022 |
0.5%, 150 mg/30 mL (5 mg/mL) |
Case Pack 2 x 25 Vials |
| 1% Lidocaine HCl Injection, USP Single Dose Teartop Vial | Vial: 0409-4279-16 Tray: 0409-4279-02 |
EG8933 | 01 Aug 2022 |
1%, 300 mg/30 mL (10 mg/mL) |
Case Pack 2 x 25 Vials |
Hospira, Inc., places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process. Hospira, Inc. has notified wholesalers, distributors, retailers, and hospitals by mail to arrange for return of any recalled product.
Wholesalers, distributors or retailers with an existing inventory of the lot, which is being recalled, should stop administration and distribution and quarantine immediately. If you have further distributed the recalled product, to the wholesale or retail level, please notify any accounts or additional locations which may have received the recalled product from you.
Hospitals/Institutions should inform Healthcare Professionals in your organization of this recall. For additional assistance, call Stericycle at 1-800-805-3093 between the hours of 8 a.m. to 5 p.m. ET, Monday through Friday.
Healthcare Professionals with questions regarding this recall can contact Pfizer using the below information.
| Contact | Contact Information | Areas of Support |
|---|---|---|
| Pfizer Medical Information | 1-800-438-1985, option 3 (9am to 5pm ET Monday through Friday) |
Medical questions regarding the product |
| Pfizer Safety | 1-800-438-1985, option 1 (24 hours a day 7 days per week) |
To report adverse events or product complaints |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088
to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Stericycle
- 1-800-805-3093
Leave a Reply