Exactech does not have, what one might call, a clean record. On the contrary, in 2008, it became apparent that the company had been involved in a kickback scheme involving surgeons throughout the country. By initiating so-called “consulting agreements” with doctors, the company essentially offered money to surgeons, who without fail pushed patients to purchase Exactech products. An investigation led by the US Attorney General’s office culminated in a $3 million settlement with the DOJ – that was in 2010.
Premarket Notification
What’s more, ever since 1994, when their Optetrak Total Knee Replacement System was first approved by the FDA, the company has continually recreated the product through a series of 510(k) premarket notifications, which, it should be known, don’t require any thorough testing. Instead, under these agreements, companies merely have to provide evidence that the product is substantially similar to its previous iterations. Thus, it’s no wonder that the product has been known to falter prematurely and has been linked to a number of severe side-effects, including a condition known as aseptic lymphocyte-dominated vasculitis-associated lesions. Patients with this condition begin to notice tumor-like structures in the skin surrounding the prosthetic. It is the result of a long-term exposure to metal.
Lawsuits Emerge
In the past few months, patients who have noticed the major faults of the Optetrak Total Knee Replacement System have started to come forward in several lawsuits against Exactech, which continues to maintain its innocence in the matter. According to one lawsuit, the company exhibited extreme negligence in choosing to release the product to the public: “Despite Defendants knowledge of early onset failures of the Optetrak knee implants and the increased risk of failure for overweight and obese patients, Defendants never alerted the FDA of the known increased risks…early onset failure mode reports are representative of the increased rate of incidents of which Defendants had become internally aware.”
The device, it should be noted, may also be linked to metallosis, a condition where tissue that has been exposed to metal necrotizes and inflames.
Marcus Talley and Loose Tibial Trays
The lawsuit, filed by Marcus Talley, alleges that the company sneakily started to withdraw the product from the market without communicating the known failures of the Optetrak System. After having total knee replacement surgery, Talley had the device implanted. And when the tibial tray, the upper part of the implant connected directly to the shin, came loose, he had to have yet another surgery to counteract the effects.
Informative Study
It’s no wonder that tibial trays have been coming loose, as subsequent tests have shown that this version of the tray – the one that was quietly recalled – is “finned” and does not bind well to the bones. What’s worse, the company should have been aware of this. According to a 2012 study, the product was deeply flawed from the beginning. The study, which monitored patients over a 25-month period, found that a number of tibial trays were on their way to coming loose and that a large portion of patients (20 percent) needed a regular dosage of painkillers. Moreover, the study concluded that the knee prosthetic yielded “poor results.”
After silently recalling the finned tray, Exactech replaced it with an updated version containing “fit” tibial trays. It remains to be seen how these will compare to the trays in question.
Just Compensation
Since the device affects nearly 500,000 people every year, we might expect more patients to come out of the woodwork seeking redress for their injuries. A company like Exactech is no stranger to faulty products. They’ve already had to recall hip and shoulder replacements. But now they may be forced to compensate those who have been injured by their alleged negligence.
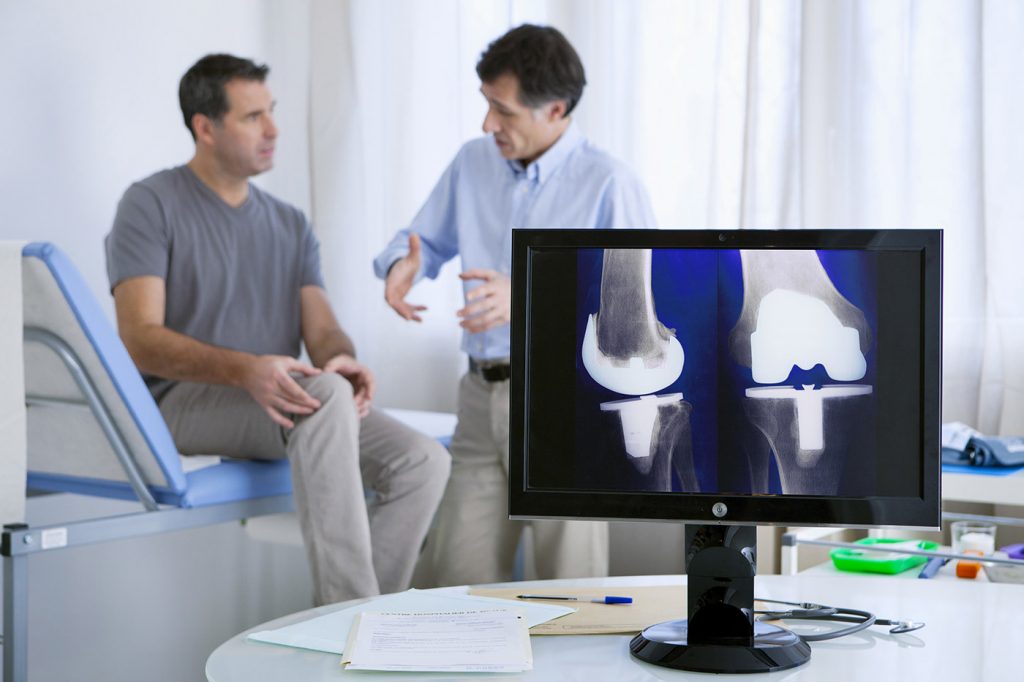
I have a Exactech Optetrak PS total knee replacement prosthesis put in Oct 2015. It has been nothing but pain. Recent test have shown is that my pain is because the devise is loosening and must come out.