On February 21, 2019, the Food and Drug Administration (FDA) posted the following urgent message on its website: “The FDA has concluded there is an increased risk of death with Uloric (febuxostat) compared to another gout medicine, allopurinol. This conclusion is based on our in-depth review of results from a safety clinical trial that found an increased risk of heart-related death and death from all causes with Uloric.”
As a result of the findings showing dangers associated with Uloric, the FDA also updated the prescribing information for this gout medication to require what’s called a “Boxed Warning,” which is the FDA’s most prominent warning. Boxed warnings are colloquially referred to as “black box warnings.” The FDA also ordered that the drug febuxostat, which is the generic name for Uloric, be used only in those patients who do not respond to allopurinol, or who experience adverse side effects when taking allopurinol. Allopurinol has been used for decades, is safe, and effective according to the FDA.
What is Uloric; What is Gout?
Approved by the FDA in 2009, Uloric is manufactured by Takeda Pharmaceuticals, one of largest pharmaceutical companies in the world and the largest drug maker in Asia. Since 2012, the company has reported earnings over $16.2 billion in revenue; Takeda Pharmaceuticals mainly produces drugs that treat metabolic disorders, gastroenterological issues, neurological issues, inflammation, and cancers.
Febuxostat works by stopping the body from turning naturally occurring purines or those that come from food and drink into uric acid. About two-thirds of the uric acid in the body is produced naturally; the rest comes from your diet, often in the form of purines, which are substances in animal and plant food that the human body converts to uric acid. Too much uric acid in the body causes gout, according to Arthritis.org.
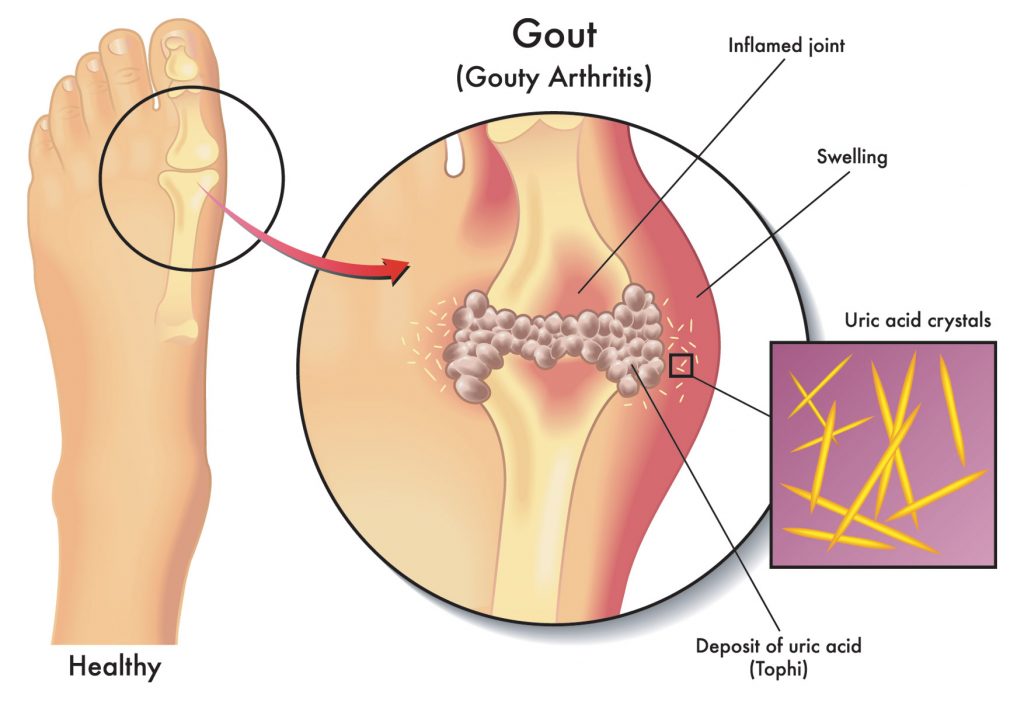
If your kidneys cannot flush uric acid from the system, it can build up in the bloodstream and be deposited as needle-shaped crystals in your joints. These crystals cause the severe inflammation and intense pain of a gout attack. MayoClinic.org explains gout as a “common and complex form of arthritis that can affect anyone.”
Gout is characterized by sudden, severe attacks of pain, swelling, redness and tenderness in the joints, often the joint at the base of the big toe. “An attack of gout can occur suddenly, often waking you up in the middle of the night with the sensation that your big toe is on fire. The affected joint is hot, swollen, and so tender that even the weight of the sheet on it may seem intolerable.”
Dangerous Drug
The FDA says there’s an increased risk of death associated with Uloric when compared to another popular gout medicine, allopurinol. Uloric has shown an increased risk of heart-related death, as well as death from other causes associated with the drug. One study showed that in patients treated with Uloric, 15 deaths from heart-related causes were observed for every 1,000 patients treated for one year. Additionally, there were 26 deaths from “any cause per 1,000 patients treated for one year with this dangerous drug.”
If you’re currently taking Uloric, please contact your doctor to find out if at risk. No drug should be stopped suddenly without first consulting the prescribing physician. Patients with the following symptoms should contact their doctor immediately:
- Chest pain
- Difficulty breathing
- Rapid or irregular heart rate
- Numbness or weakness affecting one side of the
- Dizziness, vertigo, or trouble standing
- Difficulty speaking (forming sentences)
- Severe headache that comes on quickly
Patients taking Uloric may face an increased risk of:
- Cardiac event-related death
- Non-deadly heart attack
- Non-deadly stroke
- Unstable angina
- Death
If you’ve lost a loved one who was taking Uloric, please contact a defective drug injury attorney in your area.
Leave a Reply