Summary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Potentially contain microbial contamination in the non-sterile Suturagel
- Company Name:
- Fagron Inc.
- Brand Name:
-
Brand Name(s)
- Product Description:
-
Product Description
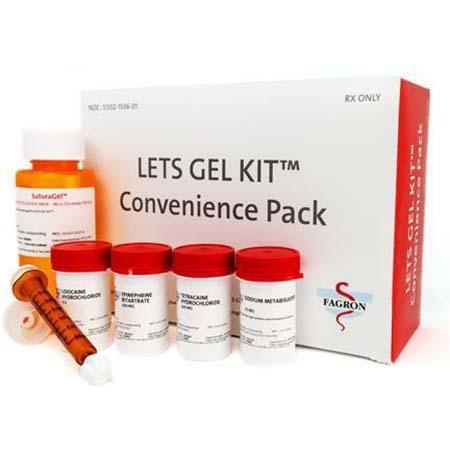
LETS GEL KIT Convenience Packs
Company Announcement
Fagron Inc. is voluntarily recalling all unexpired lots of LETS GEL KIT Convenience Packs to the hospital, pharmacy and distributor level. The product has been found to potentially contain microbial contamination in the non-sterile Suturagel Methylcellulose base component of the LETS GEL KIT Convenience Packs. FDA analysis identified Bacillus fortis/Geobacillus toebii, Bacillus spp, and Bacillus circulans as contaminants in some samples obtained during an inspection of Fagron Inc.
This non-sterile product has the potential to result in an increased chance of infection at the site of administration during open wound care. The use of this or other non-sterile products in wound treatment may increase the risk of infection, especially for immunocompromised patients, the elderly or children. To date, Fagron Inc. has not received any reports of adverse events related to this recall. Fagron Inc. places the health and well being of patients first and focuses on quality throughout our production processes. Fagron Inc. will contact all affected customers via a recall letter to arrange for the return of all products identified in the recall.
The product is used as a topical anesthetic and is packaged in boxes containing lidocaine hydrochloride, epinephrine bitartrate, tetracaine hydrochloride, sodium metabisulfite, Suturagel Methylcellulose base, adapter cap and syringes. The affected LETS GEL KIT Convenience Pack lots include the following lot numbers and expiration dates:
| Lot Number | Expiration Date |
|---|---|
| 18F25-U03-044296 | 11/8/2019 |
| 18F25-U04-044297 | 11/8/2019 |
| 18H22-U06-045284 | 1/24/2020 |
| 18I24-U05-045853 | 11/8/2019 |
| 18K06-U03-047041 | 1/24/2020 |
| 18K06-U04-047042 | 1/24/2020 |
| 18K27-U11-047460 | 3/16/2020 |
| 18K28-U10-047519 | 3/16/2020 |
| 19A06-U02-048182 | 3/16/2020 |
| 19A06-U03-048183 | 3/16/2020 |
| 19A16-U13-048363 | 3/16/2020 |
| 19A21-U02-048503 | 3/16/2020 |
| 19B10-U02-048787 | 3/16/2020 |
| 19B14-U05-048781 | 3/16/2020 |
| 19C05-U01-049199 | 3/16/2020 |
| 19C05-U02-049200 | 3/16/2020 |
| 19C11-U07-049451 | 3/16/2020 |
| 19C25-U02-048758 | 3/16/2020 |
| 19D07-U01-049884 | 3/16/2020 |
| 19D07-U02-049885 | 3/16/2020 |
| 19E09-U01-050298 | 3/16/2020 |
| 19E23-U01-050763 | 9/11/2020 |
| 19F16-U12-000052 | 9/11/2020 |
| 19F16-U13-000053 | 10/24/2020 |
| 19G07-U01-000357 | 10/24/2020 |
| 19G07-U02-000358 | 10/24/2020 |
| 19G21-U01-000930 | 10/24/2020 |
| 19G21-U02-000931 | 12/17/2020 |
| 19H01-U01-001172 | 12/17/2020 |
| 19H01-U02-001174 | 12/17/2020 |
| 19I03-U01-002019 | 2/4/2021 |
| 19I03-U02-002020 | 2/4/2021 |
| 19I03-U03-002021 | 12/17/2020 |
The product can be identified by the product packaging and labeling. The product was distributed nationwide to hospitals, pharmacies and distributors.
Fagron Inc. is notifying its distributors and customers by phone, e-mail, and/or letter and is arranging for return of all recalled products. Hospitals, pharmacies and distributors that have LETS GEL KIT Convenience Packs which are being recalled should quarantine this material. Please immediately discontinue use or distribution of the affected lots.
Consumers with questions regarding this recall can contact Stericycle via the following information.
Phone: 877-448-5308 (8am – 5pm EST)
FAX: 877-884-9410
Email: Fagron7222@stericycle.com
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using the product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Media:
- Jim Bergan
- 651-313-6536









Leave a Reply